Hiding In The Crowd: Designing Therapies To Evade Immune Detection

Using computer modeling and DNA sequencing, scientists are building better biologic medicines that are potentially invisible to the immune system.
Biologic medicines have greatly improved the lives of many patients, in particular with chronic autoimmune conditions such as rheumatoid arthritis, Crohn’s disease, multiple sclerosis and even some cancers. But the challenge with these protein-based medicines, which are made from living cells, is that some patients, over time, develop an immune response to the drug that renders it ineffective.
Timothy Hickling, an Associate Research Fellow at Pfizer’s Andover, Mass., research site, is part of a team of scientists helping to design more advanced biotherapeutic medicines that can evade detection by the immune system. “There are a variety of reasons that biologic medicines can stop working, and we know that an immune response is one of the main reasons that treatments fail,” says Hickling. “We’re working to design drugs that are stealthy, that can get in and do their work without an immune response,” says Hickling.
In many ways, it’s the opposite challenge of creating vaccines, says Hickling, who began his career as a vaccine immunologist. When designing a vaccine, scientists need to create an antigen that the immune system can recognize and develop antibodies against to provide future immune protection. “We’re using the same science as vaccines, but we’re trying to make these biologic molecules invisible,” he says.
Another piece of the puzzle Hickling and his team are working to decipher is better predicting which patients may have an immune response to a drug. “A patient could last a few months on a drug or even a couple of years without an immune response. One of the things we’d like to better understand is why some people have an immune response and some people don’t,” he says.
Biologic: Friend or Foe?
Many biologic medicines are monoclonal antibodies (mAb), which are lab-produced antibodies, or proteins, that can bind to a target in the body to help fight disease. To treat rheumatoid arthritis, mAbs block the activity of cytokines — proteins secreted by immune cells that in this case cause inflammation. For cancer therapy, mAbs can either block abnormal proteins in cancer cells or be used to boost the immune system’s response against the cancer cells.
The earliest biologic medicines were made from mouse cells, but these rodent derived protein products were often highly “immunogenic,” meaning the immune system could easily recognize it as foreign and mount a response. With advances in DNA sequencing technology, scientists began to develop “chimeric” and “humanized” biologics that contained human protein sequences to make them appear more like the antibodies produced naturally in the human body. “We’re trying to take it a step further to have ‘ultra-humanized’ biologics that can blend into the crowd and be camouflaged,” says Hickling.
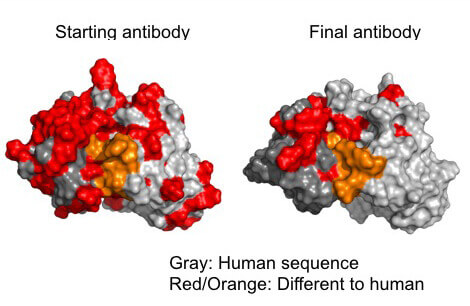
Scientists can engineer antibodies to be more stealthy by locating and removing epitopes which would be recognized as foreign. (gray/ human epitopes; red and orange/ foreign epitopes) (image credit: Timothy Hickling)
How to Blend Into the Crowd
Using computer modeling software and DNA sequencing, Hickling and his team are working to understand which parts of the biologic medicines are provoking an immune response. These medicines, which are made of antibodies, contain sections on their surface known as epitopes that the immune system’s T- and B-cells can recognize. “That’s the part of the drug that looks foreign to the immune system,” says Hickling. “We’ve been focusing a lot on T-cell responses, because these cells play a strong role in the generation of anti-drug antibodies,” he adds.
When a biotherapeutic is in its development stage, Hickling and his team use computer software to predict which parts of the molecule are most likely to appear as T-cell epitopes. “We then try to change them to different amino acid sequences that might not be recognized by the immune system,” he said. But this tweaking can be a balancing act. “We still need the drug to bind really well to its target in the body,” he says.
Once they’ve altered the structure of the drug molecule, they test it in human blood samples to measure the activity of immune cells. “This tells us that the T-cells of the immune system are responding,” he adds.
Computer Predictions for Better Outcomes
Hickling and his team are not just involved in the design stage of biologic medicines. Once a drug candidate has reached the clinical trial phase, they provide information that helps predict which drugs are going to provoke an immune response. “We use computer-based methods combining all these bits of data with the hope to predict the outcomes in clinical trials,” he says.
But Hickling adds that his team’s work is just one piece of the puzzle in understanding the immune responses to medicine. There are various other factors that trigger immune reactions to drugs. “We’re hoping to alter the way a drug is made very early on. So that it’s baked into the design.”
