Inside the Toxicology Lab: From Guardians of the Genome to Bottleneck Breakers and Beyond
When it comes to creating new medicines, the stories that most often reach the public are the discoveries of new biological pathways or novel compounds. But in the nearly decade-long journey a medicine takes to get from the lab to patients, drug safety studies are an integral, though often behind-the-scenes, part of the process. We recently spoke with Pfizer scientists who work in various areas of toxicology to learn more about the critical jobs they do to get new medicines to patients. Here are some surprising things we learned.
Protecting your DNA: Genetic Toxicology is a sub-discipline within the field that studies whether a chemical or medicine has the potential to damage a person’s DNA, causing mutations that can lead to cancer. “We start our tests very early on in the drug discovery process,” says Krista Dobo, a Senior Director in Genetic Toxicology at Pfizer’s Groton, Connecticut research site. “If a chemical has the potential to be a carcinogen, then we won’t develop it further. You want to screen for that very early on, so we don’t waste a lot of time and resources,” she says. Before a drug can be tested in humans, her team must submit evidence to the FDA that it’s not mutagenic. Because Dobo and her team work to ensure potential medicines don’t harm a person’s DNA, they sometimes jokingly refer to themselves as “Guardians of the Genome.”
Making an exception for cancer: Some oncology compounds, by their nature, are meant to damage DNA to kill cancerous cells. “In this case, then that’s accepted because the benefits to the patient outweigh the risk of not taking the medication.” For these compounds, Dobo and her team still study its effects on cellular DNA. “Our tests can help scientists understand what their compounds are doing.”
Inspired by a potato chip: Scientists like Dobo use two main types of tests to see if a drug is potentially carcinogenic. The first, the Ames Assay, was developed by biochemist Bruce Ames in the 1970s after he scanned the ingredient list on a box a potato chips and wondered if any of its preservatives might be damaging to DNA. Hoping to find a quick answer, he developed a cheap test using mutated salmonella bacteria that could act as “sensor” in the presence of DNA damaging substances. If the bacteria multiplied in the presence of the chemical, it would be a sign of a carcinogen. Ames’ work showed that cigarettes cause cancer, as well as a flame-retardant chemical once used in children’s pajamas. His test continues to be the gold standard today.
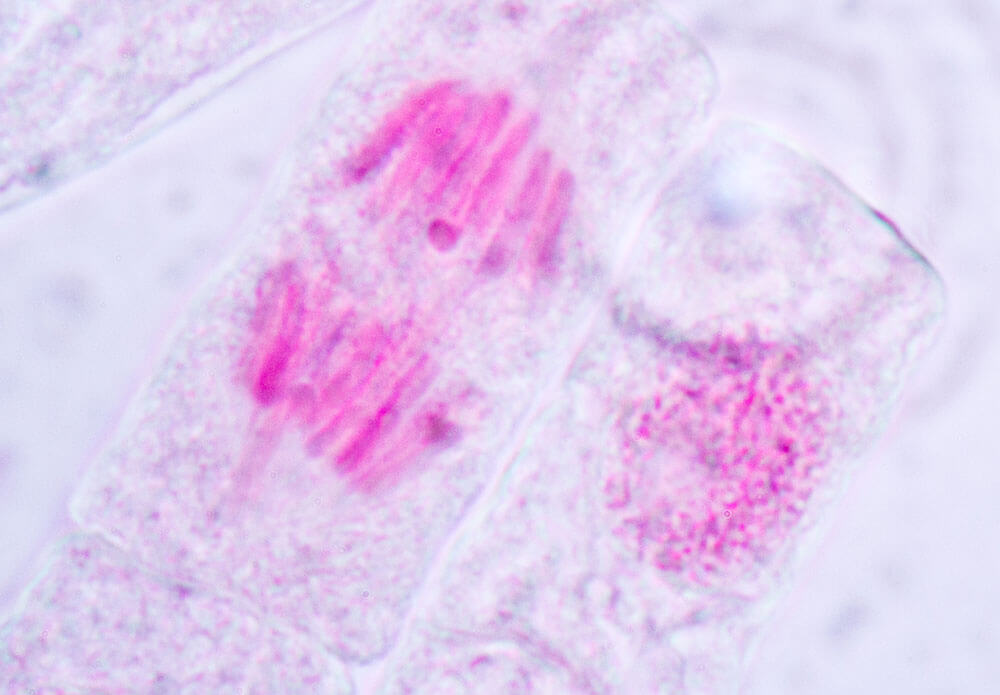
Detecting broken bits of DNA: The other main genotoxicity test screens for micronuclei, or broken bits of DNA. Within a cell, its DNA is normally contained in the nucleus. But if DNA is damaged and does not correctly repair itself, during part of the cell division process these bits of micronuclei will appear outside the nucleus. “We look at immortalized mammalian cell lines treated with chemicals to see if any micronuclei form,” says Dobo.
Saving some valuable compounds: Genetic toxicology is not only about detecting hazards. Dobo and her team also do tests to help scientists better understand how chemicals effect cell division. “A lot of the chemicals in our compound library interfere with the cell cycle,” says Dobo. “We’ve been able to continue working with chemicals that we might have otherwise thrown away, because we’ve been able to quickly tell colleagues why an abnormality is observed and whether it’s relevant or not to living organisms.”
Predicting problems before they happen: Safety pharmacology is a sub-discipline of drug safety that seeks to detect whether a new drug could damage organ systems before its tested in humans. The main areas of focus are the cardiovascular, respiratory and nervous systems. “Safety pharmacology became established in the ‘90s as a specialty area to really understand the potential safety liability at pharmacology doses,” says David Ackley, Senior Director Head of Global Safety Pharmacology at Pfizer, also based in Groton. Safety pharmacologists use a variety of high-throughput screening methods that make use of cellular models.
Inspecting the compound library: Part of the work that Ackley’s team does is studying some four million compounds in Pfizer’s liquid library to learn about potential toxic side effects on organs. “We have a group that runs hundreds of compounds a week,” says Ackley. “And that information feeds forward, so when a compound may be picked as a drug candidate, we already have a core battery of work to support it.”
Staying out of the cloud: Recently, his team has been using AI to predict the dose at which drugs can cause arrhythmia, an irregular heartbeat in patients. Specifically, they’re using machine learning to predict the concentration at which a drug may inhibit the three main ion channels responsible for electrical impulses in the heart. Once they predict that dangerous concentration for each of these channels, they plot each point on a 3D graph, creating a zone that resembles a cloud. “You want to try to stay out of the cloud,” says Ackley.
Ensuring safety during pregnancy: Developmental and reproductive toxicology (DART) seeks to determine if a drug will have any effects on a developing fetus or a person’s fertility. Most of these tests are done on animal models and are required for the registration of a new drug. The first trimester in pregnancy is a particularly critical time, as cells are rapidly dividing and exchanging signals, says Sarah Campion, Senior Principal Scientist at Pfizer, also based in Groton. “The goal of our studies is to identify any potential risks to a developing fetus in order to communicate those risks and prevent any potential birth defects,” adds Campion.
