New Hope for a Once Neglected Disease: Advances in Sickle Cell Treatments
In the early 2000s, Kelly Knee was in graduate school at Wesleyan University in Middletown, Conn. studying Molecular Biophysics. And at the time, she found it nearly impossible to secure grant funding for research on sickle cell disease, a rare blood disorder that historically has been a neglected area of medical research. But hearing stories from patients suffering from severe pain episodes and frequent hospitalizations kept her going.
“I’ve been working on this since I started graduate school," says Knee, now a senior principal scientist in Pfizer’s Rare Disease Research Unit, based in Kendall Square, Cambridge, Massachusetts. "It has been a bit of a moral imperative to see if we can help these patients."
At Pfizer, Knee is a part of a team of experts and scientists who share her goals. In 2017, the U.S Food and Drug Administration (FDA) approved two new drugs to help treat sickle cell disease, the first in nearly 20 years.1 They have since approved one more.2 Knee and her colleagues are now bringing two new potential sickle cell drugs into clinical testing with the intent to further expand treatment alternatives for sickle cell patients. “Our hope is that there will be options so that physicians can have a suite of medicines from which to select the most effective way to treat a patient,” says Knee.
Swimming against the tide
Sickle cell disease affects an estimated 100,000 people in the United States. Today, this blood-related condition affects approximately one in every 365 Black or African-American births.3
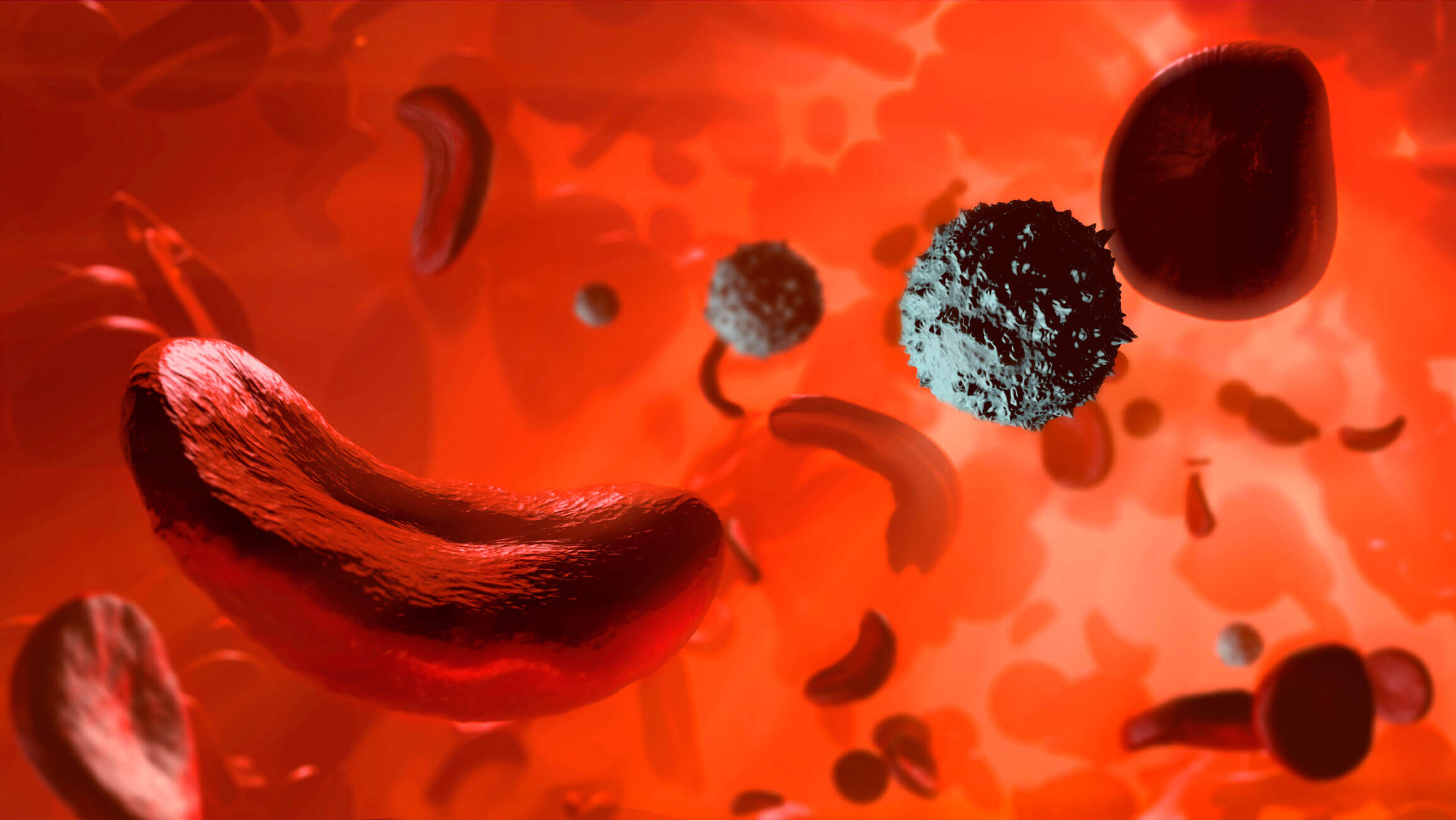
People with sickle cell disease have what are called atypical hemoglobin molecules, proteins that carry oxygen in red blood cells, which can cause the shape of red blood cells to be distorted.4 When the atypical hemoglobin molecule is not carrying oxygen—called the deoxy form—it can polymerize, forming long fibers that cause red blood cells to take on a sickle shape, or a C-shape. These distorted red blood cells can then clump in blood vessels and prematurely break down, causing anemia, fatigue, swelling, and severe pain.5,6
While scientists have long understood hemoglobin’s critical role in the condition, historically they have not targeted it with medicines, because it is such an abundant protein in the blood and therefore hard to treat effectively. Any target that requires a large dose of a drug comes with added risk. “We were swimming against the tide,” says Knee. “A lot of people in the industry have said, ‘You can't do that. That's ridiculous. There's too much hemoglobin, you'll never get it to work.’”
Reaching more patients
Despite these challenges, scientists at Pfizer have recently developed a medicine that can stabilize the oxygen-carrying form of hemoglobin so that it can outnumber the deoxy form that causes the sickling in red blood cells. “The idea is to shift the ratio of the two different types of hemoglobin, so patients would have less of the deoxy form to polymerize,” says Knee.
As the potential new drug prepares to enter clinical evaluation, Knee is especially hopeful about one important aspect of the new drug, now in clinical evaluation: The potential medicine is a small molecule making it possible to deliver in pill form, offering more convenience for patients. “Because it’s oral and shelf-stable, it’s an option that should more easily make it to patients in developing regions of the world,” she says.
Next wave of therapies
Not only do patients with sickle cell disease live with anemia and ongoing fatigue, but they can experience extremely painful episodes known as vaso-occlusive crises, which often require them to be hospitalized.7 To help alleviate this burden, Pfizer is also developing a new anti-inflammatory biologic, or protein-based medicine, that is designed to lessen the frequency of these episodes.8
The anti-inflammatory biologic targets E-selectin, an adhesion molecule that helps blood cells stick to the walls of blood vessels and to each other, an important part of wound healing and fighting infections. For patients with sickle cell disease, there is an increase in these molecules, which can cause red blood cells to clump in blood vessels and block blood flow, leading to debilitating pain episodes.
“The sickling of red blood cells is what triggers inflammation and the increase of the E-selectin molecules,” says Janice Chin, an associate research fellow in Pfizer’s Rare Disease Research Unit, also based in Cambridge. “We’re hoping with our potential medicine that we can decrease the clumping of cells and the resulting pain episodes.”
And since the three potential new medicines target different drivers of the disease, scientists see the possibility for combining them for more benefit. “It may provide patients with more options for treatments,” says Chin.
After participating in a 2020 sickle cell disease awareness walk, Knee connected with patients who are excited about the potential for new medicines.
“These patients know that developing medicines takes time and that it’s extremely hard,” says Knee, “but they’re excited that we care enough to work on potential new treatments."
--
References:
1. FDA.gov. The FDA Encourages New Treatments for Sickle Cell Disease. Available at: https://www.fda.gov/consumers/consumer-updates/fda-encourages-new-treatments-sickle-cell-disease. Accessed 6/15/2021.
2. FDA.gov. FDA approves novel treatment to target abnormality in sickle cell disease. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-novel-treatment-target-abnormality-sickle-cell-disease. Accessed 6/15/2021.
3. CDC.gov. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/ncbddd/sicklecell/data.html. Accessed 6/15/2021.
4. NHLBI.NIH.gov. Sickle Cell Disease. National Heart, Lung, and Blood Institute. Available at: https://www.nhlbi.nih.gov/health-topics/sickle-cell-disease. Accessed 6/15/2021.
5. Henry, Eric, Cellmer, Troy, Dunkelberger, Emily, et al. Allosteric control of hemoglobin S fiber formation by oxygen and its relation to the pathophysiology of sickle cell disease. Proceedings of the National Academy of Sciences: Jun 2020. Available at: https://www.pnas.org/content/117/26/15018. Accessed 6/15/2021.
6. MedlinePlus.gov. Sickle Cell Disease. Available at: https://medlineplus.gov/sicklecelldisease.html. Accessed 6/15/2021.
7. Manwani, D, Frenette, PS. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood: 2013. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3854110/. Accessed 6/15/2021.
8. Yale, Steven, Nagib, Nahed, Guthrie, Troy. Am Fam Physician: Mar 2000. Available at: https://www.aafp.org/afp/2000/0301/p1349.html. Accessed 6/15/2021.
![]()
